Step By Step CGM Guide
Here’s a step-by-step guide to getting started with CGM devices in your practice.
GUIDELINE: According to the American Diabetes Association’s "Standards of Care in Diabetes—2024," the following individuals would benefit from routine use of continuous glucose monitoring (CGM):
- Real-time CGM (rtCGM) [Recommendation 7.14, grade A] or intermittently scanned CGM (isCGM) [Recommendation 7.14, grade B] should be offered for diabetes management in adults with diabetes on multiple daily injections (MDI) or continuous subcutaneous insulin infusion (CSII) who are capable of using the devices safely (either by themselves or with a caregiver).
- rtCGM [Recommendation 7.15, grade A] or isCGM [Recommendation 7.15, grade B] should be offered for diabetes management in adults with diabetes on basal insulin who are capable of using the devices safely (either by themselves or with a caregiver).
- rtCGM [Recommendation 7.16, grade A] or isCGM [Recommendation 7.16, grade E] should be offered for diabetes management in youth with type 1 diabetes on MDI or CSII who are capable of using the devices safely (either by themselves or with a caregiver).
- rtCGM or isCGM should be offered for diabetes management in youth with type 2 diabetes on MDI or CSII who are capable of using the devices safely (either by themselves or with a caregiver). [Recommendation 7.17, grade E]
In addition, the following are recommended:
- In people with diabetes on MDI or CSII, rtCGM devices should be used as close to daily as possible for maximal benefit. [Recommendation 7.18, grade A] isCGM devices should be scanned frequently, at a minimum once every 8 hours to avoid gaps in data. [Recommendation 7.18, grade A]
- People with diabetes should have uninterrupted access to their supplies to minimize gaps in CGM. [Recommendation 7.18, grade A]
- Periodic use of rtCGM or isCGM or use of professional CGM can be helpful for diabetes management in circumstances where consistent use of CGM is not desired or available. [Recommendation 7.20, grade C]
- Skin reactions, either due to irritation or allergy, should be assessed and addressed to aid in successful use of devices. [Recommendation 7.21, grade E]
- People who wear CGM devices should be educated on potential interfering substances and other factors that may affect accuracy. [Recommendation 7.22, grade C]
American Diabetes Association Professional Practice Committee; 7. Diabetes Technology: Standards of Care in Diabetes—2024. Diabetes Care 1 January 2024; 47 (Supplement_1): S126–S144. https://pubmed.ncbi.nlm.nih.gov/38078575/
According to the “American Association of Clinical Endocrinology Clinical Practice Guideline: The Use of Advanced Technology in the Management of Persons with Diabetes Mellitus,” established clinical targets should be used to individualize glycemic targets and adjust therapy based on each individual’s overall health status, concomitant medical condition (eg, pregnancy, frailty), and risk for hypoglycemia. [AACE Recommendation 1.1.1; Grade C; Low-Intermediate Strength of Evidence1]
All Persons With Diabetes
- Number of days of active CGM use: 14 days preferred
- Percentage of data available from active CGM use: > 70% of data from 14 days
- Mean glucose: Individualized to targets
- Glucose Management Indicator (GMI): Individualized to targets
- Glycemic variability, %CV: ≤ 36%
T1D/T2D
- %Time in Range (TIR) 70 to 180 mg/dL:>70%
- %Time Below Range (TBR)<70 mg/dL:<4%
- %TBR<54 mg/dL:< 1%
- %Time Above Range (TAR)>180 mg/dL:< 25%
- %TAR>250 mg/dL:< 5%
Older/High Risk T1D/T2D
- %TIR 70 to 180 mg/dL: > 50%
- %TBR < 70 mg/dL: < 1%
- %TBR < 54 mg/dL: ~0%
- %TAR > 250 mg/dL: < 10%
Two metrics, %TIR and %TBR, should be used as a starting point for the assessment of quality of glycemic control and as the basis for therapy adjustment, with emphasis on reducing %TBR when the percentages of CGM values falling below 54 or 70 mg/dL are close to or exceed targets. [AACE Recommendation 1.1.2; Grade B; Low-Intermediate Strength of Evidence1]
- rtCGM should be recommended over isCGM to persons with diabetes with problematic hypoglycemia (frequent/severe hypoglycemia, nocturnal hypoglycemia, hypoglycemia unawareness) who require predictive alarms/alerts. However, the lifestyle of persons with diabetes and other factors should also be considered. [AACE Recommendation 2.3.1; Grade B; Intermediate Strength of Evidence1]
- When used as an adjunct to preprandial and postprandial BGM, CGM can help to achieve A1C targets in diabetes and pregnancy. [ADA Recommendation 7.19, grade B]
isCGM should be considered for persons with diabetes who meet 1 or more of the following criteria:
- Newly diagnosed with T2D
- Treated with nonhypoglycemic therapies
- Motivated to scan device several times per day
- At low risk for hypoglycemia, although desire more data than SMBG provides [AACE Recommendation 2.3.2; Grade D; Low Strength of Evidence/Expert Opinion of Task Force1]
- In patients on multiple daily injections and continuous subcutaneous insulin infusion, rtCGM devices should be used as close to daily as possible for maximal benefit. IsCGM devices should be scanned frequently, at a minimum once every 8 hours. [ADA Recommendation 7.11; Level of Evidence A2]
Grunberger G, et al. American Association of Clinical Endocrinology Clinical Practice Guideline: The Use of Advanced Technology in the Management of Persons with Diabetes Mellitus. Endocrine Practice. 2021;27:505-537. https://pubmed.ncbi.nlm.nih.gov/34116789/
CGM devices are either owned by the user for personal use or owned by the health care center for professional use. Clinic-based and owned CGM devices (Professional CGM) are placed on the patient in the provider’s office and used on a short-term basis (~3–14 days). Glucose data may be blinded or visible to the person wearing the device. At the end of the recording period, the device is returned to the clinic, glucose data are downloaded, and the information is reviewed with the patient, providing insights to help inform diabetes management.
Personal CGM devices are owned by the person with diabetes and can be used either continuously or intermittently. Patients use the information in real time to make decisions about their diabetes management. Using data collected from the personal CGM, patients together with their diabetes care team can retrospectively review the Ambulatory Glucose Profile (AGP) Report, identify any problematic glycemic patterns, discuss potential contributing factors and potential solutions, and develop an action plan to improve diabetes management.
- Diagnostic/professional CGM should be used in the management of persons with diabetes who meet 1 or more of the following criteria:
- Newly diagnosed with diabetes mellitus
- Not using CGM
- May have problematic hypoglycemia, but no access to personal CGM
- Persons with T2D treated with non-insulin therapies who would benefit from an episodic use of CGM as an educational tool
- Persons who would like to learn more about CGM before committing to daily use
- Importantly, in those using “masked” or “blinded” diagnostic/professional CGM, they must have and continue using adjunctive SMBG to assist in daily diabetes self-care.
- [AACE Recommendation 2.4.1; Grade B; Intermediate Strength of Evidence1]
- Use of professional CGM and/or intermittent rt-or isCGM can be helpful in identifying and correcting patterns of hyper- and hypoglycemia and improving A1C levels in people with diabetes on noninsulin as well as basal insulin regimens. [ADA Recommendation 7.13; Level of Evidence C2]
- Intermittent/occasional CGM may be recommended for the management of persons with diabetes who are reluctant or unable to commit to routine CGM use. [AACE Recommendation 2.5.1; Grade C; Intermediate Strength of Evidence1]
Safety Considerations
- Skin reactions, either due to irritation or allergy, should be assessed and addressed to aid in successful use of devices. [ADA Recommendation 7.14; Level of Evidence E2]
- With the use of CGM, clinicians should make a reasonable effort to ascertain that a person with diabetes is not inadvertently ingesting a substance or medication that will cause the CGM to deliver false or misleading information. Furthermore, clinicians should make a reasonable effort to make persons with diabetes aware of the theoretical risk of radiation exposure to diabetes technologies. [AACE Recommendation 3.1.1; Grade C; Low Strength of Evidence/Expert Opinion of Task Force1]
- Persons with diabetes who have a care provider, such as a spouse, adult child of a geriatric person with diabetes, or parent of a child with diabetes, who remotely monitors glucose data, should be cautioned that remote glucose monitoring is dependent upon server functionality and that data interruption can result. Back-up plans of having persons with diabetes revert to SMBG or methods to communicate CGM data to those who remotely follow will be needed until functionality can be restored. [AACE Recommendation 3.1.2; Grade D; Low Strength of Evidence/Expert Opinion of Task Force1]
In addition, the following are recommended:
- The continuation of CGM and/or CSII (insulin pump, SAP, LGS/PLGS) should be considered in hospitalized persons with diabetes without cognitive impairment and ideally with the presence of a family member who is knowledgeable and educated in the use of these devices or with a specialized inpatient diabetes team available for advice and support. [AACE Recommendation 2.10.1; Grade A; Intermediate Strength of Evidence1]
- rtCGM is recommended for persons ≥ 65 years old with insulin-requiring diabetes to achieve improved glycemic control, reduce episodes of severe hypoglycemia, and improve QoL. However, glycemic goals should be individualized due to increased comorbidities and reduced capacity to detect and counter-regulate against severe hypoglycemia in this population. [AACE Recommendation 2.10.2; Grade A; Intermediate-High Strength of Evidence1]
- Telemedicine, including periodic phone calls, smartphone-web interactions, and periodic supervision by health care professional interactions, is strongly recommended to treat persons with diabetes, provide diabetes education, remotely monitor glucose and/or insulin data to indicate the need for therapy adjustments, and improve diabetes-related outcomes/control with better engagement. [AACE Recommendation 2.11.1; Grade A; Intermediate-High Strength of Evidence1]
- Initiation and use of diabetes technology should be implemented by health care professionals who are trained, committed, and experienced to prescribe and direct the use of these tools. Clinicians should have the infrastructure to support the needs of persons with diabetes using the technology. [AACE Recommendation 4.1.1; Grade B; Intermediate Strength of Evidence/Expert Opinion of Task Force1]
Steps to Developing a Personal CGM Program
 Identify the feasibility of the program through evaluation of your population and of support structures within your practice setting by asking yourself these questions.
Identify the feasibility of the program through evaluation of your population and of support structures within your practice setting by asking yourself these questions.
- Are you currently doing all that you can to reduce your patients’ HbA1C levels and optimize their diabetes management?
- How could personal CGM benefit your patient population?
- Would your patient population be amenable to personal CGM?
- Are the providers in your practice resourced appropriately to support a personal CGM program?
- What does your practice hope to gain by implementing this program?
- How could a personal CGM program add value to your patients, providers, and practice?
- Could it help improve the diabetes care provided in your practice?
- Could it help improve the diabetes metrics in your practice?
 Step 1 Checklist:
Step 1 Checklist:
- Categorize your diabetes population by types of diabetes therapies they utilize.
- What percent uses multiple daily insulin injections or insulin pumps?
- What percent uses basal insulin plus orals or other injectables?
- What percent uses oral agents that may cause hypoglycemia, such as sulfonylureas, etc.?
- How many are currently utilizing personal CGM devices?
- Meet with the members of your healthcare team to identify pros and cons of using personal CGM to manage people with diabetes in your practice.
- Meet with all important stakeholders (leadership team, support staff, etc.) to identify any concerns they have and obtain their buy-in.
- Identify outcomes of a successful Personal CGM Program to people with diabetes, the providers, and the practice.
 TIP: Reach out to colleagues at other practices with an already established Personal CGM Program and network with them about what worked and what didn’t go as hoped when getting the program started. Continue to utilize such contacts as a resource as you implement your program. Professional CGM implementation can be an important steppingstone for preparation of personal CGM implementation.
TIP: Reach out to colleagues at other practices with an already established Personal CGM Program and network with them about what worked and what didn’t go as hoped when getting the program started. Continue to utilize such contacts as a resource as you implement your program. Professional CGM implementation can be an important steppingstone for preparation of personal CGM implementation.
 Adding a Personal CGM Program to your practice will require changes to your current workflow, and having enthusiastic and well-qualified team members will help ensure a successful launch of the program.
Adding a Personal CGM Program to your practice will require changes to your current workflow, and having enthusiastic and well-qualified team members will help ensure a successful launch of the program.
- Identify who will be on the team and what their roles will be.
- A diabetes educator/certified diabetes educator (CDE) can be extremely valuable to the team, if you have one on your staff.
- A pharmacist can provide medication-related expertise, which can add value to a Personal CGM Program because they can work with the patient and physician to make adjustments to the treatment plan based on CGM results.
- A person that is knowledgeable downloading CGM devices will be vital to the team.
- The CGM device company representative may be part of your team to assist with device training and follow-up.
- It is helpful to define the Personal CGM process from start to finish so that you have a clear understanding of all steps.
 Step 2 Checklist:
Step 2 Checklist:
- Identify the members of your team, and define the roles and responsibilities of each team member.
- Meet as a team regularly, and seek ongoing feedback from all involved parties.
- Define the workflow of an in-person visit from beginning to end:
- Seek ongoing feedback from all involved parties
- Include specifics about patient selection, use of the device, providing education to patient, diagnosis/coding/billing, documentation, downloading and interpreting results.
- Define the workflow of a remote monitoring visit from beginning to end.
- Update existing protocols or create new ones to accommodate this new workflow in daily practice.
- Plan for enhanced staffing levels as you roll out the new program.
- Identify necessary resources that will support the new workflow (e.g., a designated computer and/or printer).
- Solicit staff feedback every step of the way.
- Plan for ongoing scheduled evaluation of workflow and adjust as needed.
- Determine what data you will want to collect ongoing.
- Seek ongoing ideas from all and don’t be averse to changing direction if warranted.
 TIP: Actively seek to minimize disruption to the current workflow. Anticipate barriers and be prepared to address them. It is important to consider all perspectives when designing the workflow, including that of the person with diabetes, the support staff, and providers. You may want to include a patient representative to assist with the design, implementation, and evaluation of the workflow to ensure that you fully understand their perspective.
TIP: Actively seek to minimize disruption to the current workflow. Anticipate barriers and be prepared to address them. It is important to consider all perspectives when designing the workflow, including that of the person with diabetes, the support staff, and providers. You may want to include a patient representative to assist with the design, implementation, and evaluation of the workflow to ensure that you fully understand their perspective.
 Commercial insurance and Medicaid plans vary regarding amount of coverage for personal CGM devices and their ongoing supplies. Defining the documentation and billing process before you begin will help ensure that all potential roadblocks are anticipated, identified, and addressed.
Commercial insurance and Medicaid plans vary regarding amount of coverage for personal CGM devices and their ongoing supplies. Defining the documentation and billing process before you begin will help ensure that all potential roadblocks are anticipated, identified, and addressed.
Medicare will cover personal CGM if the following criteria are met:
- The beneficiary has diabetes mellitus; and,
- The beneficiary is insulin-treated or experiences problematic hypoglycemia, and,
- The beneficiary’s insulin treatment regimen requires frequent adjustment by the beneficiary on the basis of BGM or CGM testing results; and,
- Within six (6) months prior to ordering the CGM, the treating practitioner has an in-person visit with the beneficiary to evaluate their diabetes control and determined that criteria (1-3) above are met; and,
- Every six (6) months following the initial prescription of the CGM, the treating practitioner has an in-person visit with the beneficiary to assess adherence to their CGM regimen and diabetes treatment plan.
- Problematic hypoglycemia is defined as recurrent level 2 hypoglycemia (glucose <54mg/dL) and documentation of 1 or more previous medication adjustment or a level 3 hypoglycemia which includes a glucose <54mg/dL and requiring assistance from another individual.
 Step 3 Checklist:
Step 3 Checklist:
- Seek input from all team members regarding the design of the workflow.
- Define the workflow of an in-person visit from beginning to end.
- Educate staff on the billing codes to utilize for Personal CGM.
- Map out the documentation and billing process before you begin to help to ensure that all involved staff understands the necessary codes to bill when there is a patient visit with a personal CGM to be evaluated.
- Identify the team member to be accountable for coordination of insurance benefits and requirements.
- Provide an in-service for staff on coding, billing, and documentation requirements for Personal CGM.
- Provide an in-service for staff about CGM remote monitoring visits that includes documentation and billing requirements.
- Identify team member(s) to perform ongoing documentation, coding, and billing audits of individuals in the program.
 TIP: You may be requested to do a peer-to-peer review to further determine the medical necessity for the personal CGM. It may be helpful to have a template prepared that includes details that you want to include in a review. (Open new page or build into accordion: https://pro.aace.com/cgm/toolkit/billing-codes)
TIP: You may be requested to do a peer-to-peer review to further determine the medical necessity for the personal CGM. It may be helpful to have a template prepared that includes details that you want to include in a review. (Open new page or build into accordion: https://pro.aace.com/cgm/toolkit/billing-codes)
Billing Codes for Personal and Professional Continuous Glucose Monitoring Visits and Services
CPT Code
Type of Service
Provider
Frequency
Type of Visit
95249
Personal CGM Start-up and Training Ambulatory CGM of interstitial tissue fluid via a subcutaneous sensor for a minimum of 72 hours; patient provided equipment, sensor placement, hook-up, calibration of monitor, patient training, and printout of recording
RN, PharmD/RPh, RD, CDE, or MA (if within their scope of practice) and billed by the supervising physician, advanced practitioner, or hospital outpatient department
Once for the lifetime of the personal CGM device
Face-to-face visit
95250
Professional CGM Ambulatory CGM of interstitial tissue fluid via a subcutaneous sensor for a minimum of 72 hours; physician or other qualified health care professional (office) provided equipment, sensor placement, hook-up, calibration of monitor, patient training, removal of sensor, and printout of recording
RN, PharmD/RPh, RD, CDE, or MA (if within their scope of practice) and billed by the supervising physician, advanced practitioner, or hospital outpatient department
Maximum of once per month
Face-to-face visit
95251
CGM Interpretation Ambulatory CGM of interstitial tissue fluid via a subcutaneous sensor for a minimum of 72 hours; analysis, interpretation, and report
Physician (MD, DO), NP, PA, or clinical nurse specialist
Maximum of once per month
Not required to have a face-to-face visit
–25 modifier
Evaluation and Management (Separate Identifiable Service) An E/M CPT code can be billed on the same day as codes 95249, 95250, and/or 95251 if documentation supports the medical necessity of a significant and separately identifiable evaluation and management service performed the same date. Modifier 25 is added to the E/M code to report a significant and separately identifiable evaluation and management performed above the CGM services.
Physician (MD, DO), NP, PA, or clinical nurse specialist
With office visits
Face-to-face visit
 Staff will need to be trained on all available personal CGM program options. Training should cover an overview of the workflow, including patient selection, use of the device, providing education to the patient, diagnosis/coding/billing, documentation, download, and interpretation of results. In addition, all team members are aware of their roles and responsibilities. Proper staff preparation will help ensure that the staff is on board with the program and motivated to put in their best effort to help make it a success.
Staff will need to be trained on all available personal CGM program options. Training should cover an overview of the workflow, including patient selection, use of the device, providing education to the patient, diagnosis/coding/billing, documentation, download, and interpretation of results. In addition, all team members are aware of their roles and responsibilities. Proper staff preparation will help ensure that the staff is on board with the program and motivated to put in their best effort to help make it a success.
 Step 4 Checklist:
Step 4 Checklist:
- To compare various FDA-approved Personal and Professional CGM systems, consult this AACE CGM Device Comparison page.
- Review the guidelines above identifying which individuals would most likely benefit from Personal CGM.
- Refer to a vendor’s training site to view training and set-up information for a device. Or set up vendor training sessions for all involved staff to learn about the different personal CGM devices and how to download information. Consult the following individual CGM device websites for training information.
- Abbott FreeStyle Libre (Libra 3, Libra 2, and Libra 14 day)
- Abbott FreeStyle Healthcare Provider Resources
- Dexcom CGM Education and Use
- Dexcom CGM Healthcare Provider Resources
- Medtronic Guardian Connect System User Guide
- Medtronic CGM Healthcare Provider Resources
- Senseonics Eversense CGM User Guides
- Senseonics Eversense CGM Healthcare Provider Resources
- Provide resources for staff about the different routes to obtain Personal CGM (how to pick up the product from the pharmacy or obtain from DME supplier).
- Develop staff competencies .that are role specific and identify a process to review and document their skills on an ongoing basis.
- Review the AGP Guideline below.
- Review these resources: the Ambulatory Glucose Profile, Interpreting how to interpret Interpreting AGP Reports, and Sample AGP Reports.
- Set up provider training on program workflow and protocols, diagnosis/coding/billing, interpretation, and documentation of downloaded information.
- Consider trying the program out with just a few people with personal CGM devices at first, as a test pilot. This will help to identify any process issues.
- Plan for annual training refresher.
- Host a program launch kick-off event to review roles/responsibilities and provide a program overview.
- Identify staff ‘superusers’ who can serve as resources to others.
- Have vendor and IT available during implementation.
- Make device procedure reference materials and troubleshooting guides widely available.
- Provide a forum for staff to provide feedback and suggestions.
- Keep an open communication line with the team members and involved staff so the team feels this is a collective effort.
GUIDELINE: Ambulatory Glucose Profile (AGP) may be utilized to assess glycemic status in persons with diabetes. [AACE Recommendation 2.2.1; Grade B; Low Strength of Evidence1]
When using AGP, a systematic approach to interpret CGM data is recommended:
- Review overall glycemic status (e.g., GMI, average glucose).
- Check TBR, TIR, and TAR statistics, focusing on hypoglycemia (TBR) first. If the TBR statistics are above the cut-point for the clinical scenario (ie, for most with T1D > 4% < 70 mg/dL; > 1% < 54 mg/dL), the visit should focus on this issue. Otherwise, move on to the TIR and TAR statistics.
- Review the 24-hour glucose profile to identify the time(s) and magnitude(s) of the problem identified
- Review treatment regimen and adjust as needed. [AACE Recommendation 2.2.2; Grade B; Low Strength of Evidence1]
 TIP: Providing thorough pre-implementation training will help ensure a smooth start to the program. Solicit staff feedback frequently during and after the training sessions. Consider recording the training sessions so that new staff in the future will have access to it.
TIP: Providing thorough pre-implementation training will help ensure a smooth start to the program. Solicit staff feedback frequently during and after the training sessions. Consider recording the training sessions so that new staff in the future will have access to it.
 The Personal CGM Program will anticipate an upfront learning curve for the person with diabetes.
The Personal CGM Program will anticipate an upfront learning curve for the person with diabetes.
- It is vitally important to provide initial and ongoing education about their personal CGM device.
 Step 5 Checklist:
Step 5 Checklist:
- Review handouts with the person with diabetes and discuss how to get the most out of their Personal CGM. The power of data can help the person feel more in control of their diabetes and can facilitate a more collaborative approach with the healthcare team.
- A library of helpful handouts can be found on the Tools and Resources page of the Association of Diabetes Care & Education Specialists (ADCES) website.
- Provide initial training, including a detailed education plan for the patient about their personal CGM device and ongoing training to help them maximize the use of their device.
- Use this AACE Action Plan as a helpful resource.
- Identify procedures to provide ongoing support for a person using Personal CGM.
- Consult the individual CGM device websites listed in Step 4 for device-specific information and training resources.
 TIP: Make the person with diabetes the centerpiece of the program. Spend time up front on education and support to ensure their understanding of the process and goals of the program. Provide ongoing support as they continue their CGM journey.
TIP: Make the person with diabetes the centerpiece of the program. Spend time up front on education and support to ensure their understanding of the process and goals of the program. Provide ongoing support as they continue their CGM journey.
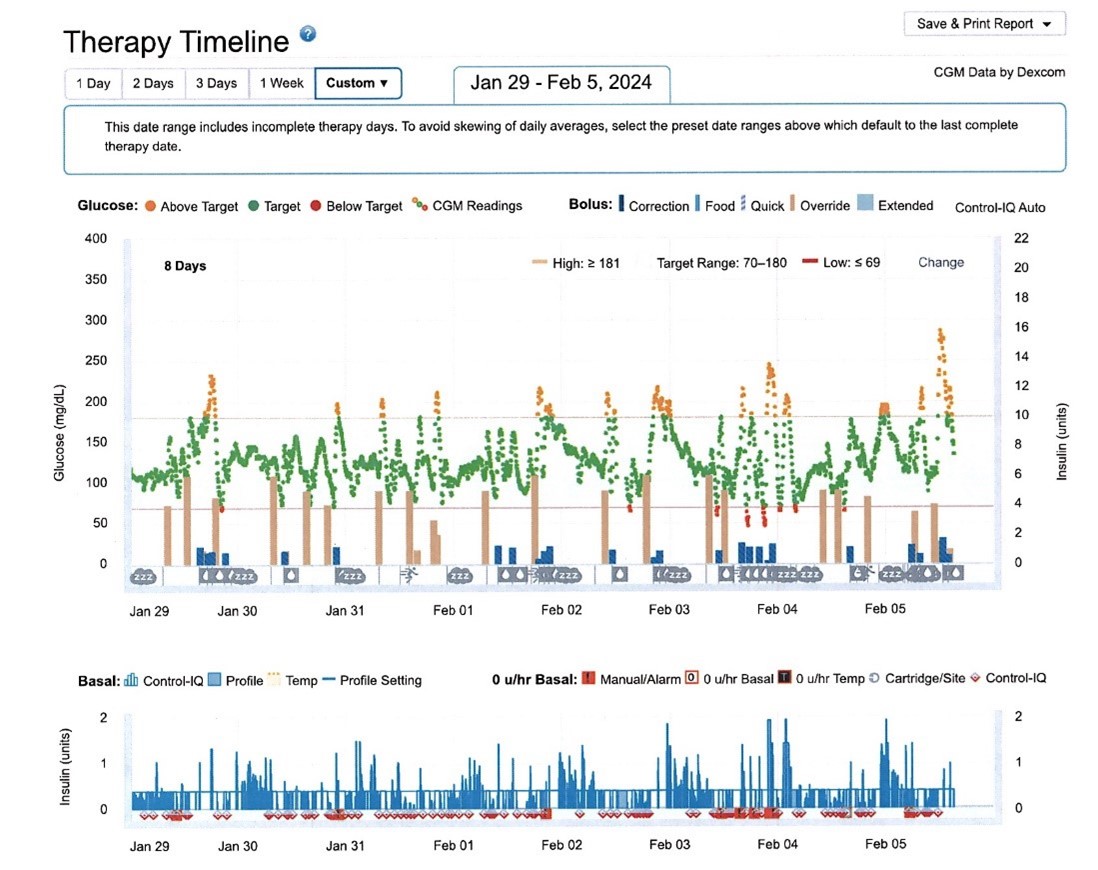
 You are now ready to try out your new program!
You are now ready to try out your new program!
- Solicit feedback and ideas from staff and patients throughout the implementation process.
- Start tracking success measures at program initiation.
 Step 6 Checklist:
Step 6 Checklist:
- Walk through the process from start to finish.
- Provide on-site technical and product support resources to staff during launch.
- Perform frequent check-ins with staff at this beginning phase of the program implementation.
- Schedule a weekly staff meeting to discuss how things are going.
- Start collecting any clinical and financial data that you will want to analyze.
 TIP: If possible, try to have some cushion in your staffing during this time. This will enable staff to take the time necessary to fully learn and practice the new procedures and processes. Communicate frequently with support staff to identify any challenges or obstacles to program success. Utilize vendor support and IT support to ensure a smooth implementation.
TIP: If possible, try to have some cushion in your staffing during this time. This will enable staff to take the time necessary to fully learn and practice the new procedures and processes. Communicate frequently with support staff to identify any challenges or obstacles to program success. Utilize vendor support and IT support to ensure a smooth implementation.
 Program evaluation is an ongoing process.
Program evaluation is an ongoing process.
- Be thoughtful and quickly responsive to suggestions/ideas/feedback.
- Refer often to the metrics of program success that you have identified.
- Practice continuous quality improvement.
- Planning for the future of your Personal CGM program is a crucial component of a successful program. Use these questions to guide future planning:
- What are the next steps for your program?
- Do you want to establish a satellite location?
- Do you want to start working with patients remotely?
- Do you want to offer a Personal CGM support group or annual group education class geared to people who use personal CGM devices?
- Do you want to share your program experience and lessons learned with other care teams?
 Step 7 Checklist:
Step 7 Checklist:
- Gather staff feedback on a consistent basis.
- Survey the persons with diabetes that are being served by this program regarding their satisfaction.
- Analyze and evaluate clinical and financial data that you have collected.
- Compare diabetes outcomes post-Personal CGM Program to pre-program outcomes.
- If you are not meeting success metrics, reevaluate your program process and amend as necessary.
- If you are meeting success metrics, disseminate the results widely.
- Meet with the entire team to determine next steps. Is the program the right size? Does it have the right composition of staff? Is there reasonable access to the program?
- Hold a meeting with the entire team to discuss future goals for the program.
- Explore feasibility of the goals.
- Market the program to others that may wish to refer to your program.
 TIP: Establish a timeline for ongoing program evaluation. A program may not attain the expected measures of success in the first few rounds of evaluation. Staff will become more efficient with program processes as time goes by. Ease of use will improve, and staff confidence and competence will show ongoing improvements as well.
TIP: Establish a timeline for ongoing program evaluation. A program may not attain the expected measures of success in the first few rounds of evaluation. Staff will become more efficient with program processes as time goes by. Ease of use will improve, and staff confidence and competence will show ongoing improvements as well.
CGM Resources for Primary Care Professionals
- Wondering whether to refer your patient to an endocrinologist? Continuous glucose monitoring (CGM) technology allows patients to track their blood sugar levels over time. Listen to AACE Podcast Episode 22 to hear from Endocrinologist Cheryl R. Rosenfeld, DO, FACE, FACP, FSVM, ECNU, and Internal Medicine Specialist Angela Cavanna, DO, as they discuss the benefits of CGM and different scenarios when you may want to refer a patient to an endocrinologist.
- Learn more about diabetes care and other endocrinology topics. For more in-depth information on all of our disease state resources, listen to AACE Podcasts now. Our podcasts cover a wide range of endocrine science, including novel treatment recommendations, discussion regarding our clinical guidelines, and much more
References
This content has been adapted from the Association of Diabetes Care & Education Specialists, Professional Continuous Glucose Monitoring Implementation Playbook, and the Personal Continuous Glucose Monitoring Implementation Playbook. Playbooks are available for download at here.
Note: These Playbooks include Worksheet Resources that support these step-by-step processes.
Selected Literature
- Aleppo G. Clinical application of time in range and other metrics. Diabetes Spectr. 2021;34:109-118. https://pubmed.ncbi.nlm.nih.gov/34149251/
- Aleppo G, Webb K. Continuous glucose monitoring integration in clinical practice: a stepped guide to data review and interpretation. J Diabetes Sci Technol. 2019;13:664-673. https://pubmed.ncbi.nlm.nih.gov/30453772/
- American Diabetes Association Professional Practice Committee; 7. Diabetes Technology: Standards of Care in Diabetes—2024. Diabetes Care 1 January 2024; 47 (Supplement_1): S126–S144. https://pubmed.ncbi.nlm.nih.gov/38078575/
- Battelino T, et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations From the International Consensus on Time in Range. Diabetes Care. 2019;42:1593-1603. https://pubmed.ncbi.nlm.nih.gov/31177185/
- Beck RW, et al. The relationships between time in range, hyperglycemia metrics, and HbA1C. J Diabetes Sci Technol. 2019;13:614-626. https://pubmed.ncbi.nlm.nih.gov/30636519/
- Beck RW, et al; DIAMOND Study Group. Continuous Glucose Monitoring Versus Usual Care in Patients With Type 2 Diabetes Receiving Multiple Daily Insulin Injections: A Randomized Trial. Ann Intern Med. 2017 Sep 19;167(6):365-374. https://pubmed.ncbi.nlm.nih.gov/28828487/
- Bergenstal RM, et al. Glucose management indicator (GMI): a new term for estimating A1C from continuous glucose monitoring. Diabetes Care. 2018;41:2275-2280. https://pubmed.ncbi.nlm.nih.gov/30224348/
- Carlson AL, et al. Clinical use of continuous glucose monitoring in adults with type 2 diabetes. Diabetes Technol Ther. 2017;19(Suppl. 2):S4–S11. https://pubmed.ncbi.nlm.nih.gov/28541137/
- Danne T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40:1631-1640. https://pubmed.ncbi.nlm.nih.gov/29162583/
- Ehrhardt NM, et al. The effect of real-time continuous glucose monitoring on glycemic control in patients with type 2 diabetes mellitus. J Diabetes Sci Technol. 2011;5:668-675. https://pubmed.ncbi.nlm.nih.gov/21722581/
- Grunberger G, et al. American Association of Clinical Endocrinology Clinical Practice Guideline: The Use of Advanced Technology in the Management of Persons with Diabetes Mellitus. Endocrine Practice. 2021;27:505-537. https://pubmed.ncbi.nlm.nih.gov/34116789/
- Hack T, et al. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8:55–73. https://pubmed.ncbi.nlm.nih.gov/28000140/
- Kruger DF, et al. Reference guide for integrating continuous glucose monitoring into clinical practice. Diabetes Educ. 2019;45:3S-20S. https://pubmed.ncbi.nlm.nih.gov/30541402/
- Maiorino MI, et al. Effects of Continuous Glucose Monitoring on Metrics of Glycemic Control in Diabetes: A Systematic Review With Meta-analysis of Randomized Controlled Trials. Diabetes Care. 2020 May;43(5):1146-1156. https://pubmed.ncbi.nlm.nih.gov/32312858/
- Martens T, et al. MOBILE Study Group. Effect of Continuous Glucose Monitoring on Glycemic Control in Patients With Type 2 Diabetes Treated With Basal Insulin: A Randomized Clinical Trial. JAMA. 2021 Jun 8;325(22):2262-2272. https://pubmed.ncbi.nlm.nih.gov/34077499/
- Miller EM. Using continuous glucose monitoring in clinical practice. Clin Diabetes. 2020 Dec;38(5):429-438. https://pubmed.ncbi.nlm.nih.gov/33384468/
- Riddlesworth TD, et al. Optimal sampling duration for continuous glucose monitoring to determine long-term glycemic control. Diabetes Technol Ther. 2018;20:314-316. https://pubmed.ncbi.nlm.nih.gov/29565197/
- Ruedy KJ, et al. DIAMOND Study Group. Continuous glucose monitoring in older adults with type 1 and type 2 diabetes using multiple daily injections of insulin: results from the DIAMOND trial. J Diabetes Sci Technol. 2017;11:1138-1146. https://pubmed.ncbi.nlm.nih.gov/28449590/
- Shrivastav M, et al. Type 2 diabetes management in primary care: the role of retrospective, professional continuous glucose monitoring. Diabetes Sprect. 2018;31:279-287. https://pubmed.ncbi.nlm.nih.gov/30140145/
- Siegmund T, et al. Discrepancies Between Blood Glucose and Interstitial Glucose-Technological Artifacts or Physiology: Implications for Selection of the Appropriate Therapeutic Target J Diabetes Sci Technol. 2017;11:766‐772. https://pubmed.ncbi.nlm.nih.gov/28322063/
- Sierra JA, et al. Clinical and economic benefits of professional CGM among people with type 2 diabetes in the united states: analysis of claims and lab data. J Med Econ. 2018;21:225-230. https://pubmed.ncbi.nlm.nih.gov/28994334/
- Wright EE, et al. Use of Flash Continuous Glucose Monitoring Is Associated With A1C Reduction in People With Type 2 Diabetes Treated With Basal Insulin or Noninsulin Therapy. Diabetes Spect. 2021;34:184-189. https://pubmed.ncbi.nlm.nih.gov/34149259/
- Glossary https://pro.aace.com/cgm/toolkit/glossary
